Exubera for Treatment of Diabetes - Exubera Full Prescribing Information
Brand Name: Exubera
Generic Name: insulin human
Dosage Form: inhalation powder
Contents:
Description
Clinical Pharmacology
Clinical Studies
Indications and Usage
Contraindications
Warnings
Precautions
Drug Interactions
Adverse Reactions
Overdosage
Dosage and Administration
How Supplied
Exubera, insulin human [rDNA origin] Patient information (in plain English)
Description
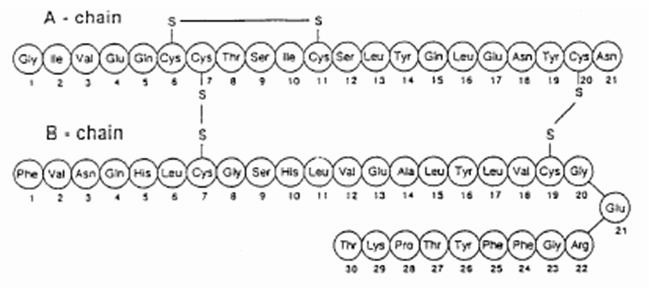
Exubera® consists of blisters containing human insulin inhalation powder, which are administered using the Exubera® Inhaler. Exubera blisters contain human insulin produced by recombinant DNA technology utilizing a non-pathogenic laboratory strain of Escherichia coli (K12). Chemically, human insulin has the empirical formula C257H383N65O77S6 and a molecular weight of 5808. Human insulin has the following primary amino acid sequence:
Exubera (insulin human [rDNA origin]) Inhalation Powder is a white to off-white powder in a unit dose blister (fill mass, see Table 1). Each unit dose blister of Exubera contains a 1 mg or 3 mg dose of insulin (see Table 1) in a homogeneous powder formulation containing sodium citrate (dihydrate), mannitol, glycine, and sodium hydroxide. After an Exubera blister is inserted into the inhaler, the patient pumps the handle of the inhaler and then presses a button, causing the blister to be pierced. The insulin inhalation powder is then dispersed into the chamber, allowing the patient to inhale the aerosolized powder.
Under standardized in vitro test conditions, Exubera delivers a specific emitted dose of insulin from the mouthpiece of the inhaler (see Table 1). A fraction of the total particle mass is emitted as fine particles capable of reaching the deep lung. Up to 45% of the 1 mg blister contents, and up to 25% of the 3 mg blister contents, may be retained in the blister.
Table 1: Dose Nomenclature and Information
| Fill Mass (mg powder) | Nominal Dose (mg insulin) | Emitted Dose*,†(mg insulin) | Fine Particle Dosec,†(mg insulin) |
|---|---|---|---|
| |||
| 1.7 | 1.0 | 0.53 | 0.4 |
| 5.1 | 3.0 | 2.03 | 1.0 |
The actual amount of insulin delivered to the lung will depend on individual patient factors, such as inspiratory flow profile. In vitro, emitted aerosol metrics are unaffected at flow rates above 10 L/min.
Clinical Pharmacology
Mechanism of Action
The primary activity of insulin is regulation of glucose metabolism. Insulin lowers blood glucose concentrations by stimulating peripheral glucose uptake by skeletal muscle and fat, and by inhibiting hepatic glucose production. Insulin inhibits lipolysis in the adipocyte, inhibits proteolysis, and enhances protein synthesis.
Pharmacokinetics
Absorption
Exubera delivers insulin by oral inhalation. The insulin is absorbed as quickly as subcutaneously administered rapid-acting insulin analogs and more quickly than subcutaneously administered regular human insulin in healthy subjects and in patients with type 1 or type 2 diabetes (see Figure 1).
Figure 1: Mean Changes in Free Insulin Serum Concentrations ( µU/mL) in Patients with Type 2 Diabetes Following Administration of Single Doses of Inhaled Insulin from Exubera (6 mg) and Subcutaneous Regular Human Insulin (18U)
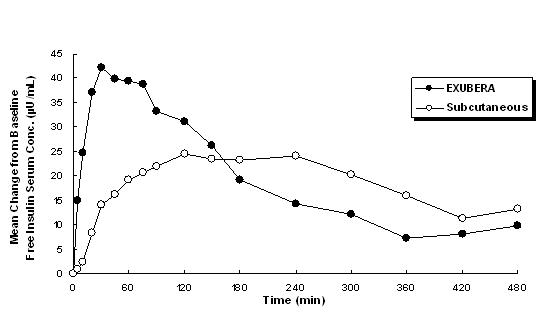
In clinical studies in patients with type 1 and type 2 diabetes, after inhalation of Exubera, serum insulin reached peak concentration more quickly than after subcutaneous injection of regular human insulin, 49 minutes (range 30 to 90 minutes) compared to 105 minutes (range 60 to 240 minutes), respectively.
In clinical studies, the absorption of subcutaneous regular human insulin declined with increasing patient body mass index (BMI). However, the absorption of insulin following inhalation of Exubera was independent of BMI.
In a study in healthy subjects, systemic insulin exposure (AUC and Cmax) following administration of Exubera increased with dose over a range of 1 to 6 mg when administered as combinations of 1 and 3 mg blisters.
In a study where the dosage form of three 1 mg blisters was compared with one 3 mg blister, Cmax and AUC after administration of three 1 mg blisters were approximately 30% and 40% greater, respectively, than that after administration of one 3 mg blister (see DOSAGE AND ADMINISTRATION).
Distribution and Elimination
Because recombinant human insulin is identical to endogenous insulin, the systemic distribution and elimination are expected to be the same. However, this has not been confirmed for Exubera.
Pharmacodynamics
Exubera, like subcutaneously administered rapid-acting insulin analogs, has a more rapid onset of glucose-lowering activity than subcutaneously administered regular human insulin. In healthy volunteers, the duration of glucose-lowering activity for Exubera was comparable to subcutaneously administered regular human insulin and longer than subcutaneously administered rapid-acting insulin analogs (see Figure 2).
Figure 2. Mean Glucose Infusion Rate (GIR) Normalized to GIRmax for Each Subject Treatment Versus Time in Healthy Volunteers
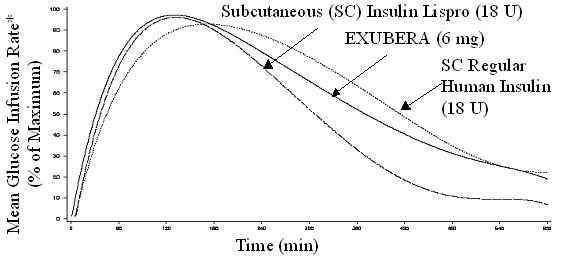
*Determined as amount of glucose infused to maintain constant plasma glucose concentrations, normalized to maximum values (percent of maximum values); indicative of insulin activity.
When Exubera is inhaled, the onset of glucose-lowering activity in healthy volunteers occurs within 10-20 minutes. The maximum effect on glucose lowering is exerted approximately 2 hours after inhalation. The duration of glucose-lowering activity is approximately 6 hours.
In patients with type 1 or type 2 diabetes, Exubera has a greater glucose-lowering effect within the first two hours after dosing when compared with subcutaneously administered regular human insulin.
The intra-subject variability of glucose-lowering activity of Exubera is generally comparable to that of subcutaneously administered regular human insulin in patients with type 1 and 2 diabetes.
Special Populations
Pediatric Patients
In children (6-11 years) and adolescents (12-17 years) with type 1 diabetes, time to peak insulin concentration for Exubera was achieved faster than for subcutaneous regular human insulin, which is consistent with observations in adult patients with type 1 diabetes.
Geriatric Patients
There are no apparent differences in the pharmacokinetic properties of Exubera when comparing patients over the age of 65 years and younger adult patients.
Gender
In subjects with and without diabetes, no apparent differences in the pharmacokinetic properties of Exubera were observed between men and women.
Race
A study was performed in 25 healthy Caucasian and Japanese non-diabetic subjects to compare the pharmacokinetic and pharmacodynamic properties of Exubera, versus subcutaneous injection of regular human insulin. The pharmacokinetic and pharmacodynamic properties of Exubera were comparable between the two populations.
Obesity
The absorption of Exubera is independent of patient BMI.
Renal Impairment
The effect of renal impairment on the pharmacokinetics of Exubera has not been studied. Careful glucose monitoring and dose adjustments of insulin may be necessary in patients with renal dysfunction (see PRECAUTIONS, Renal Impairment).
Hepatic Impairment
The effect of hepatic impairment on the pharmacokinetics of Exubera has not been studied. Careful glucose monitoring and dose adjustments of insulin may be necessary in patients with hepatic dysfunction (see PRECAUTIONS).
Pregnancy
The absorption of Exubera in pregnant patients with gestational and pre-gestational type 2 diabetes was consistent with that in non-pregnant patients with type 2 diabetes (see PRECAUTIONS).
Smoking
In smokers, the systemic insulin exposure for Exubera is expected to be 2 to 5 fold higher than in non-smokers. Exubera is contraindicated in patients who smoke or who have discontinued smoking less than 6 months prior to starting Exubera therapy. If a patient starts or resumes smoking, Exubera must be discontinued immediately due to the increased risk of hypoglycemia, and an alternative treatment must be utilized (see CONTRAINDICATIONS).
In clinical studies of Exubera in 123 patients (69 of whom were smokers), smokers experienced a more rapid onset of glucose-lowering action, greater maximum effect, and a greater total glucose-lowering effect (particularly during the first 2-3 hours after dosing), compared to non-smokers.
Passive Cigarette Smoke
In contrast to the increase in insulin exposure following active smoking, when Exubera was administered to 30 healthy non-smoking volunteers following 2 hours of exposure to passive cigarette smoke in a controlled experimental setting, insulin AUC and Cmax were reduced by approximately 20% and 30%, respectively. The pharmacokinetics of Exubera have not been studied in nonsmokers who are chronically exposed to passive cigarette smoke.
Patients with Underlying Lung Diseases
The use of Exubera in patients with underlying lung disease, such as asthma or COPD, is not recommended because the safety and efficacy of Exubera in this population have not been established (see WARNINGS). The use of Exubera is contraindicated in patients with unstable or poorly controlled lung disease, because of wide variations in lung function that could affect the absorption of Exubera and increase the risk of hypoglycemia or hyperglycemia (see CONTRAINDICATIONS).
In a pharmacokinetic study in 24 non-diabetic subjects with mild asthma, the absorption of insulin following administration of Exubera, in the absence of treatment with a bronchodilator, was approximately 20% lower than the absorption seen in subjects without asthma. However, in a study in 24 non-diabetic subjects with Chronic Obstructive Pulmonary Disease (COPD), the systemic exposure following administration of Exubera was approximately two-fold higher than that in normal subjects without COPD (see PRECAUTIONS).
Administration of albuterol 30 minutes prior to administration of Exubera in non-diabetic subjects with both mild asthma (n=36) and moderate asthma (n=31) resulted in a mean increase in insulin AUC and Cmax of between 25 and 50% compared to when Exubera was administered alone (see PRECAUTIONS).
Clinical Studies
The safety and efficacy of Exubera has been studied in approximately 2500 adult patients with type 1 and type 2 diabetes. The primary efficacy parameter for most studies was glycemic control, as measured by the reduction from baseline in hemoglobin A1c (HbA1c).
Type 1 Diabetes
A 24-week, randomized, open-label, active-control study (Study A) was conducted in patients with type 1 diabetes to assess the safety and efficacy of Exubera administered pre-meal three times daily (TID) with a single nighttime injection of Humulin® U Ultralente® (human insulin extended zinc suspension) (n = 136). The comparator treatment was subcutaneous regular human insulin administered twice daily (BID) (pre-breakfast and pre-dinner) with BID injection of NPH human insulin (human insulin isophane suspension) (n = 132). In this study, the mean age was 38.2 years (range: 20-64) and 52% of the subjects were male.
A second 24-week, randomized, open-label, active-control study (Study B) was conducted in patients with type 1 diabetes to assess the safety and efficacy of Exubera (n = 103) compared to subcutaneous regular human insulin (n = 103) when administered TID prior to meals. In both treatment arms, NPH human insulin was administered BID (in the morning and at bedtime) as the basal insulin. In this study, the mean age was 38.4 years (range: 19-65) and 54% of the subjects were male.
In each study, the reduction in HbA1c and the rates of hypoglycemia were comparable for the two treatment groups. Exubera-treated patients had a greater reduction in fasting plasma glucose than patients in the comparator group. The percentage of patients reaching an HbA1c level of <8% (per American Diabetes Association treatment Action Level at the time of study conduct) and an HbA1c level of <7% was comparable between the two treatment groups. The results for Studies A and B are shown in Table 2.
Table 2: Results of Two 24-Week, Active-Control, Open-Label Trials in Patients With Type 1 Diabetes (Studies A and B)
| Study A | Study B | |||
|---|---|---|---|---|
| Exubera (TID) + UL (QD) | SC R (BID) + NPH (BID) | Exubera (TID) + NPH (BID) | SC R (TID) + NPH (BID) | |
| Sample Size | 136 | 132 | 103 | 103 |
| UL = Humulin® U Ultralente®; SC R = subcutaneous regular human insulin | ||||
| ||||
| HbA1c (%) | ||||
| Baseline mean | 7.9 | 8.0 | 7.8 | 7.8 |
| Adj. mean change from baseline | -0.2 | -0.4 | -0.3 | -0.2 |
| Exubera minus SC R* | 0.14 | -0.11 | ||
| 95% CI for treatment difference | (-0.03, 0.32) | (-0.30, 0.08) | ||
| Fasting Plasma Glucose (mg/dL) | ||||
| Baseline mean | 191 | 198 | 178 | 191 |
| Adj. mean change from baseline | -32 | -6 | -23 | 13 |
| Exubera minus SC R | -27 | -35 | ||
| 95% CI for treatment difference | (-47, -6) | (-58, -13) | ||
| 2-hr Post-Prandial Glucose Concentration (mg/dL) | ||||
| Baseline mean | 283 | 305 | 273 | 293 |
| Adj. mean change from baseline | -21 | 14 | -1 | -3 |
| Exubera minus SC R | -35 | 2 | ||
| 95% CI for treatment difference | (-61, -8) | (-29, 32) | ||
| Patients with end-of-study HbA1c < 8%†| 64.0% | 68.2% | 74.8% | 66.0% |
| Patients with end-of-study HbA1c < 7% | 16.9% | 19.7% | 28.2% | 30.1% |
| Body Weight | ||||
| Baseline mean (kg) | 77.4 | 76.4 | 76.0 | 76.9 |
| Adj. mean change from baseline (kg) | 0.4 | 1.1 | 0.4 | 0.6 |
| Exubera minus SC R | -0.72 | -0.24 | ||
| 95% CI for treatment difference | (-1.48, 0.04) | (-1.07, 0.59) | ||
| End of study daily insulin dose | ||||
| Short-acting insulin | 13.4 mgc | 18.3 IU | 10.9 mgc | 25.7 IU |
| Long-acting insulin | 26.4 IU | 37.1 IU | 31.5 IU | 31.9 IU |
Type 2 Diabetes
Monotherapy in Patients Not Optimally Controlled With Diet and Exercise Treatment
A 12-week, randomized, open-label, active-control study (Study C) was conducted in patients with type 2 diabetes not optimally controlled with diet and exercise, assessing the safety and efficacy of pre-meal TID Exubera (n = 75) compared to an insulin-sensitizing agent. In this study, the mean age was 53.7 years (range: 28-80), 55% of the subjects were male and the mean body mass index was 32.3 kg/m2.
At 12 weeks, HbA1c levels in patients treated with Exubera decreased 2.2% (SD = 1.0) from a baseline of 9.5% (SD = 1.1). The proportion of patients treated with Exubera reaching an end-of-study HbA1c level of <8% increased to 82.7%. The proportion of patients treated with Exubera reaching an end-of-study HbA1c level of
Monotherapy and Add-On Therapy in Patients Previously Treated With Oral Agent Therapy
A 12-week, randomized, open-label, active-control study (Study D) was conducted in patients with type 2 diabetes who were currently receiving treatment, but were poorly controlled, with two oral agents (OA). Baseline OAs included an insulin secretagogue, and either metformin or a thiazolidinedione. Patients were randomized to one of three arms: continuing OA therapy alone (n = 96), switching to pre-meal TID Exubera monotherapy (n = 102) or adding pre-meal TID Exubera to continued OA therapy (n = 100). In this study, the mean age was 57.4 years (range: 33-80), 66% of the subjects were male and the mean body mass index was 30 kg/m2.
Exubera monotherapy and Exubera in combination with OA therapy were superior to OA therapy alone in reducing HbA1c levels from baseline. The rates of hypoglycemia for the two Exubera treatment groups were slightly higher than in the OA therapy alone group. Compared to OA therapy alone, the percentage of patients reaching an HbA1c level of <8% (per American Diabetes Association treatment Action Level at time of study conduct) and an HbA1c level of <7% was greater for patients treated with Exubera monotherapy and Exubera in combination with OA therapy. Patients in both Exubera treatment groups had greater reductions in fasting plasma glucose than patients treated with OA therapy alone. The results for Study D are shown in Table 3.
Table 3: Results of a 12-Week, Active-Control, Open-Label Trial in Patients With Type 2 Diabetes Not Optimally Controlled With Dual Oral Agent Therapy (Study D)
| Study D | Exubera monotherapy | OAs* | Exubera + OAs | ||
|---|---|---|---|---|---|
| Sample Size | 102 | 96 | 100 | ||
| |||||
| HbA1c (%) | |||||
| Baseline mean | 9.3 | 9.3 | 9.2 | ||
| Adj. mean change from baseline | -1.4 | -0.2 | -1.9 | ||
| Exubera group minus OAs†| -1.18†,c, § | -1.67†, ¶, § | |||
| 95% CI for treatment difference | (-1.41, -0.95) | (-1.90, -1.44) | |||
| Fasting Plasma Glucose (mg/dL) | |||||
| Baseline mean | 203 | 203 | 195 | ||
| Adj. mean change from baseline | -23 | 1 | -53 | ||
| Exubera group minus OAs | -24c | -53 ¶ | |||
| 95% CI for treatment difference | (-36, -11) | (-66, -41) | |||
| Patients with end-of-study HbA1c < 8%# | 55.9% | 18.8% | 86.0% | ||
| Patients with end-of-study HbA1c < 7% | 16.7% | 1.0% | 32.0% | ||
| Body Weight | |||||
| Baseline mean (kg) | 89.5 | 88.0 | 88.6 | ||
| Adj. mean change from baseline (kg) | 2.8 | 0.0 | 2.7 | ||
| Exubera group minus OAs | 2.80c | 2.75 ¶ | |||
| 95% CI for treatment difference | (1.94, 3.65) | (1.89, 3.61) | |||
A 24-week, randomized, open-label, active-control study (Study E) was conducted in patients with type 2 diabetes, currently receiving sulfonylurea therapy. This study was designed to assess the safety and efficacy of the addition of pre-meal Exubera to continued sulfonylurea therapy (n = 214) compared to the addition of pre-meal metformin to continued sulfonylurea therapy (n = 196). Subjects were stratified according to their HbA1c at Week -1. Two strata were defined: a low HbA1c stratum (HbA1c ≥8% to ≤9.5%) and a high HbA1c stratum (HbA1c >9.5 to ≤12%).
Exubera in combination with sulfonylurea was superior to metformin and sulfonylurea in reducing HbA1c values from baseline in the high stratum group. Exubera in combination with sulfonylurea was comparable to metformin in combination with sulfonylurea in reducing HbA1c values from baseline in the low stratum group. The rate of hypoglycemia was higher after the addition of Exubera to sulfonylurea than after the addition of metformin to sulfonylurea. The percentage of patients reaching target HbA1c values of 8% and 7% was comparable between treatment groups in both strata, as was reduction in fasting plasma glucose (see Table 4).
Another 24-week, randomized, open-label, active-control study (Study F) was conducted in patients with type 2 diabetes, currently receiving metformin therapy. This study was designed to assess the safety and efficacy of the addition of pre-meal Exubera to continued metformin therapy (n = 234) compared to the addition of pre-meal glibenclamide to continued metformin therapy (n = 222). Subjects in this study were also stratified to one of two strata as defined in Study E.
Exubera in combination with metformin was superior to glibenclamide and metformin in reducing HbA1c values from baseline and achieving target HbA1c values in the high stratum group. Exubera in combination with metformin was comparable to glibenclamide in combination with metformin in reducing HbA1c values from baseline and achieving target HbA1c values in the low stratum group. The rate of hypoglycemia was slightly higher after the addition of Exubera to metformin than after the addition of glibenclamide to metformin. Reduction in fasting plasma glucose was comparable between treatment groups (see Table 4).
Table 4: Results of Two 24-Week, Active-Control, Open-Label Trials in Patients With Type 2 Diabetes Previously On Oral Agent Therapy (Studies E and F)
| Study E | Study F | |||||||
|---|---|---|---|---|---|---|---|---|
| Exubera + SU* | Met*+ SU* | Exubera + SU* | Met*+ SU* | Exubera + Met* | Gli* + Met* | Exubera + Met* | Gli* + Met* | |
| High stratum†| Low stratum†| High stratum†| Low stratum†| |||||
| Sample Size | 113 | 103 | 101 | 93 | 109 | 103 | 125 | 119 |
| ||||||||
| HbA1c (%) | ||||||||
| Baseline mean | 10.5 | 10.6 | 8.8 | 8.8 | 10.4 | 10.6 | 8.6 | 8.7 |
| Adj. mean change from baseline | -2.2 | -1.8 | -1.9 | -1.9 | -2.2 | -1.9 | -1.8 | -1.9 |
| Exubera minus OAc | -0.38c, § | -0.07 | -0.37c, ¶ | 0.04 | ||||
| 95% CI for treatment difference | (-0.63, -0.14) | (-0.33, 0.19) | (-0.62, -0.12) | (-0.19, 0.27) | ||||
| Fasting Plasma Glucose (mg/dL) | ||||||||
| Baseline mean | 241 | 237 | 197 | 198 | 223 | 243 | 187 | 196 |
| Mean change from baseline | -46 | -47 | -48 | -52 | -42 | -40 | -46 | -49 |
| Exubera minus OA | 1 | 4 | -2 | 4 | ||||
| 95% CI for treatment difference | (-11, 12) | (-8, 16) | (-14, 10) | (-7, 15) | ||||
| Subjects with end-of-study HbA1c < 8%# | 48.7% | 44.7% | 81.2% | 73.1% | 72.5% | 56.3% | 80.8% | 86.6% |
| Subjects with end-of-study HbA1c < 7% | 20.4% | 14.6% | 30.7% | 32.3% | 33.9% | 17.5% | 40.0% | 42.9% |
| Body Weight | ||||||||
| Baseline mean (kg) | 80.8 | 79.5 | 79.9 | 81.9 | 88.3 | 87.8 | 90.3 | 88.2 |
| Adj. mean change from baseline (kg) | 3.6 | -0.0 | 2.4 | -0.3 | 2.8 | 2.5 | 2.0 | 1.6 |
| Exubera minus OA | 3.60 | 2.67 | 0.26 | 0.38 | ||||
| 95% CI for treatment difference | (2.81, 4.39) | (1.84, 3.51) | (-0.70, 1.21) | (-0.52, 1.27) | ||||
Use in Patients Previously Treated With Subcutaneous Insulin
A 24-week, randomized, open-label, active-control study (Study G) was conducted in insulin-treated patients with type 2 diabetes to assess the safety and efficacy of Exubera administered pre-meal TID with a single nighttime injection of Humulin® U Ultralente® (n = 146) compared to subcutaneous regular human insulin administered BID (pre-breakfast and pre-dinner) with BID injection of NPH human insulin (n = 149). In this study, the mean age was 57.5 years (range: 23-80), 66% of the subjects were male and the mean body mass index was 30.3 kg/m2.
The reductions from baseline in HbA1c, percent of patients reaching an HbA1c level of <8% (per American Diabetes Association treatment Action Level at time of study conduct) and an HbA1c level of <7%, as well as the rates of hypoglycemia, were similar between treatment groups. Exubera-treated patients had a greater reduction in fasting plasma glucose than patients in the comparator group. The results for Study G are shown in Table 5.
Table 5: Results of a 24-Week, Active-Control, Open-Label Trial in Patients With Type 2 Diabetes Previously Treated With Subcutaneous Insulin (Study G)
| Study G | Exubera (TID) + UL (QD) | SC R (BID) + NPH (BID) |
|---|---|---|
| Sample Size | 146 | 149 |
| UL = Humulin® U Ultralente®; SC R = subcutaneous regular human insulin | ||
| ||
| HbA1c (%) | ||
| Baseline mean | 8.1 | 8.2 |
| Adj. mean change from baseline | -0.7 | -0.6 |
| Exubera minus SC R* | -0.07 | |
| 95% CI for treatment difference | (-0.31, 0.17) | |
| Fasting Plasma Glucose (mg/dL) | ||
| Baseline mean | 152 | 159 |
| Adj. mean change from baseline | -22 | -6 |
| Exubera minus SC R | -16.36 | |
| 95% CI for treatment difference | (-27.09, -5.36) | |
| Patients with end-of-study HbA1c < 8%†| 76.0% | 69.1% |
| Patients with end-of-study HbA1c < 7% | 45.2% | 32.2% |
| Body Weight | ||
| Baseline mean (kg) | 90.6 | 89.0 |
| Adj. mean change from baseline (kg) | 0.1 | 1.3 |
| Exubera minus SC R | -1.28 | |
| 95% CI for treatment difference | (-1.96, -0.60) | |
| End of study daily insulin dose | ||
| Short-acting insulin | 16.6 mgc | 25.5 IU |
| Long-acting insulin | 37.9 IU | 52.3 IU |
Indications and Usage
Exubera is indicated for the treatment of adult patients with diabetes mellitus for the control of hyperglycemia. Exubera has an onset of action similar to rapid-acting insulin analogs and has a duration of glucose-lowering activity comparable to subcutaneously administered regular human insulin. In patients with type 1 diabetes, Exubera should be used in regimens that include a longer-acting insulin. In patients with type 2 diabetes, Exubera can be used as monotherapy or in combination with oral agents or longer-acting insulins.
Contraindications
Exubera is contraindicated in patients hypersensitive to Exubera or one of its excipients.
Exubera is contraindicated in patients who smoke or who have discontinued smoking less than 6 months prior to starting Exubera therapy. If a patient starts or resumes smoking, Exubera must be discontinued immediately due to the increased risk of hypoglycemia, and an alternative treatment must be utilized (see CLINICAL PHARMACOLOGY, Special Populations, Smoking). The safety and efficacy of Exubera in patients who smoke have not been established.
Exubera is contraindicated in patients with unstable or poorly controlled lung disease, because of wide variations in lung function that could affect the absorption of Exubera and increase the risk of hypoglycemia or hyperglycemia.
Warnings
Exubera differs from regular human insulin by its rapid onset of action. When used as mealtime insulin, the dose of Exubera should be given within 10 minutes before a meal.
Hypoglycemia is the most commonly reported adverse event of insulin therapy, including Exubera. The timing of hypoglycemia may differ among various insulin formulations.
Patients with type 1 diabetes also require a longer-acting insulin to maintain adequate glucose control.
Any change of insulin should be made cautiously and only under medical supervision. Changes in insulin strength, manufacturer, type (e.g., regular, NPH, analogs), or species (animal, human) may result in the need for a change in dosage. Concomitant oral antidiabetic treatment may need to be adjusted.
Glucose monitoring is recommended for all patients with diabetes.
Because of the effect of Exubera on pulmonary function, all patients should have pulmonary function assessed prior to initiating therapy with Exubera (see PRECAUTIONS: Pulmonary Function).
The use of Exubera in patients with underlying lung disease, such as asthma or COPD, is not recommended because the safety and efficacy of Exubera in this population have not been established (see PRECAUTIONS: Underlying Lung Disease).
In clinical trials of Exubera, there have been 6 newly diagnosed cases of primary lung malignancies among Exubera-treated patients, and 1 newly diagnosed case among comparator-treated patients. There has also been 1 postmarketing report of a primary lung malignancy in an Exubera-treated patient. In controlled clinical trials of Exubera, the incidence of new primary lung cancer per 100 patient-years of study drug exposure was 0.13 (5 cases over 3900 patient-years) for Exubera-treated patients and 0.02 (1 case over 4100 patient-years) for comparator-treated patients. There were too few cases to determine whether the emergence of these events is related to Exubera. All patients who were diagnosed with lung cancer had a prior history of cigarette smoking.
Precautions
General
As with all insulin preparations, the time course of Exubera action may vary in different individuals or at different times in the same individual. Adjustment of dosage of any insulin may be necessary if patients change their physical activity or their usual meal plan. Insulin requirements may be altered during intercurrent conditions such as illness, emotional disturbances, or stress.
Hypoglycemia
As with all insulin preparations, hypoglycemic reactions may be associated with the administration of Exubera. Rapid changes in serum glucose concentrations may induce symptoms similar to hypoglycemia in persons with diabetes, regardless of the glucose value. Early warning symptoms of hypoglycemia may be different or less pronounced under certain conditions, such as long duration of diabetes, diabetic nerve disease, use of medications such as beta-blockers, or intensified diabetes control (see PRECAUTIONS: Drug Interactions). Such situations may result in severe hypoglycemia (and, possibly, loss of consciousness) prior to patients' awareness of hypoglycemia.
Renal Impairment
Studies have not been performed in patients with renal impairment. As with other insulin preparations, the dose requirements for Exubera may be reduced in patients with renal impairment (see CLINICAL PHARMACOLOGY, Special Populations).
Hepatic Impairment
Studies have not been performed in patients with hepatic impairment. As with other insulin preparations, the dose requirements for Exubera may be reduced in patients with hepatic impairment (see CLINICAL PHARMACOLOGY, Special Populations).
Allergy
Systemic Allergy
In clinical studies, the overall incidence of allergic reactions in patients treated with Exubera was similar to that in patients using subcutaneous regimens with regular human insulin.
As with other insulin preparations, rare, but potentially serious, generalized allergy to insulin may occur, which may cause rash (including pruritus) over the whole body, shortness of breath, wheezing, reduction in blood pressure, rapid pulse, or sweating. Severe cases of generalized allergy, including anaphylactic reactions, may be life threatening. If such reactions occur from Exubera, Exubera should be stopped and alternative therapies considered.
Antibody Production
Insulin antibodies may develop during treatment with all insulin preparations including Exubera. In clinical studies of Exubera where the comparator was subcutaneous insulin, increases in insulin antibody levels (as reflected by assays of insulin binding activity) were significantly greater for patients who received Exubera than for patients who received subcutaneous insulin only. No clinical consequences of these antibodies were identified over the time period of clinical studies of Exubera; however, the long-term clinical significance of this increase in antibody formation is unknown.
Respiratory
Pulmonary Function
In clinical trials up to two years duration, patients treated with Exubera demonstrated a greater decline in pulmonary function, specifically the forced expiratory volume in one second (FEV1) and the carbon monoxide diffusing capacity (DLCO), than comparator-treated patients. The mean treatment group difference in pulmonary function favoring the comparator group, was noted within the first several weeks of treatment with Exubera, and did not change over the two year treatment period (See ADVERSE REACTIONS: Pulmonary Function).
During the controlled clinical trials, individual patients experienced notable declines in pulmonary function in both treatment groups. A decline from baseline FEV1 of ≥ 20% at last observation occurred in 1.5% of Exubera-treated and 1.3% of comparator-treated patients. A decline from baseline DLCO of ≥ 20% at last observation occurred in 5.1% of Exubera-treated and 3.6% of comparator treated patients.
Because of the effect of Exubera on pulmonary function, all patients should have spirometry (FEV1) assessed prior to initiating therapy with Exubera. Assessment of DLCO should be considered. The efficacy and safety of Exubera in patients with baseline FEV1 or DLCO < 70% predicted have not been established and the use of Exubera in this population is not recommended.
Assessment of pulmonary function (e.g., spirometry) is recommended after the first 6 months of therapy, and annually thereafter, even in the absence of pulmonary symptoms. In patients who have a decline of ≥ 20% in FEV1 from baseline, pulmonary function tests should be repeated. If the ≥ 20% decline from baseline FEV1 is confirmed, Exubera should be discontinued. The presence of pulmonary symptoms and lesser declines in pulmonary function may require more frequent monitoring of pulmonary function and consideration of discontinuation of Exubera.
Underlying Lung Disease
The use of Exubera in patients with underlying lung disease, such as asthma or COPD, is not recommended because the efficacy and safety of Exubera in this population have not been established.
Bronchospasm
Bronchospasm has been rarely reported in patients taking Exubera. Patients experiencing such a reaction should discontinue Exubera and seek medical evaluation immediately. Re-administration of Exubera requires a careful risk evaluation, and should only be done under close medical monitoring with appropriate clinical facilities available.
Intercurrent Respiratory Illness
Exubera has been administered to patients with intercurrent respiratory illness (e.g. bronchitis, upper respiratory tract infections, rhinitis) during clinical studies. In patients experiencing these conditions, 3-4% temporarily discontinued Exubera therapy. There was no increased risk of hypoglycemia or worsened glycemic control observed in Exubera-treated patients compared to patients treated with subcutaneous insulin. During intercurrent respiratory illness, close monitoring of blood glucose concentrations, and dose adjustment, may be required.
Information for Patients
Patients should be instructed on self-management procedures including glucose monitoring; proper Exubera inhalation technique; and hypoglycemia and hyperglycemia management. Patients must be instructed on handling of special situations such as intercurrent conditions (illness, stress, or emotional disturbances), an inadequate or skipped insulin dose, inadvertent administration of an increased insulin dose, inadequate food intake, or skipped meals.
Patients should be informed that in clinical studies, treatment with Exubera was associated with small, non-progressive mean declines in pulmonary function relative to comparator treatments. Because of the effect of Exubera on pulmonary function, pulmonary function tests are recommended prior to initiating treatment with Exubera. Following initiation of therapy, periodic pulmonary function tests are recommended (see PRECAUTIONS Respiratory, Pulmonary Function).
Patients should inform their physician if they have a history of lung disease, because the use of Exubera is not recommended in patients with underlying lung disease (e.g., asthma or COPD), and is contraindicated in patients with poorly controlled lung disease.
Women with diabetes should be advised to inform their doctor if they are pregnant or are contemplating pregnancy.
Drug Interactions
A number of substances affect glucose metabolism and may require insulin dose adjustment and particularly close monitoring.
The following are examples of substances that may reduce the blood glucose-lowering effect of insulin that may result in hyperglycemia: corticosteroids, danazol, diazoxide, diuretics, sympathomimetic agents (e.g., epinephrine, albuterol, terbutaline), glucagon, isoniazid, phenothiazine derivatives, somatropin, thyroid hormones, estrogens, progestogens (e.g., in oral contraceptives), protease inhibitors, and atypical antipsychotic medications (e.g., olanzapine and clozapine).
The following are examples of substances that may increase the blood glucose-lowering effect of insulin and susceptibility to hypoglycemia: oral antidiabetic products, ACE inhibitors, disopyramide, fibrates, fluoxetine, MAO inhibitors, pentoxifylline, propoxyphene, salicylates, and sulfonamide antibiotics.
Beta-blockers, clonidine, lithium salts, and alcohol may either increase or reduce the blood glucose-lowering effect of insulin. Pentamidine may cause hypoglycemia, which may sometimes be followed by hyperglycemia.
In addition, under the influence of sympatholytic medicinal products such as beta-blockers, clonidine, guanethidine, and reserpine, the signs and symptoms of hypoglycemia may be reduced or absent.
Bronchodilators and other inhaled products may alter the absorption of inhaled human insulin (see CLINICAL PHARMACOLOGY, Special Populations). Consistent timing of dosing of bronchodilators relative to Exubera administration, close monitoring of blood glucose concentrations and dose titration as appropriate are recommended.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Two-year carcinogenicity studies in animals have not been performed. Insulin was not mutagenic in the Ames bacterial reverse mutation test in the presence and absence of metabolic activation.
In Sprague-Dawley rats, a 6-month repeat-dose toxicity study was conducted with insulin inhalation powder at doses up to 5.8 mg/kg/day (compared to the clinical starting dose of 0.15 mg/kg/day, the rat high dose was 39 times or 8.3 times the clinical dose, based on either a mg/kg or a mg/m2 body surface area comparison). In Cynomolgus monkeys, a 6-month repeat-dose toxicity study was conducted with inhaled insulin at doses up to 0.64 mg/kg/day. Compared to the clinical starting dose of 0.15 mg/kg/day, the monkey high dose was 4.3 times or 1.4 times the clinical dose, based on either a mg/kg or a mg/m2 body surface area comparison. These were maximum tolerated doses based on hypoglycemia.
Compared to control animals, there were no treatment-related adverse effects in either species on pulmonary function, gross or microscopic morphology of the respiratory tract or bronchial lymph nodes. Similarly, there was no effect on cell proliferation indices in alveolar or bronchiolar area of the lung in either species.
Because recombinant human insulin is identical to the endogenous hormone, reproductive/fertility studies were not performed in animals.
Pregnancy
Teratogenic Effects
Pregnancy Category C
Animal reproduction studies have not been conducted with Exubera. It is also not known whether Exubera can cause fetal harm when administered to a pregnant woman or whether Exubera can affect reproductive capacity. Exubera should be given to a pregnant woman only if clearly needed.
Nursing Mothers
Many drugs, including human insulin, are excreted in human milk. For this reason, caution should be exercised when Exubera is administered to a nursing woman. Patients with diabetes who are lactating may require adjustments in Exubera dose, meal plan, or both.
Pediatric Use
Long-term safety and effectiveness of Exubera in pediatric patients have not been established (see CLINICAL PHARMACOLOGY, Special Populations).
Geriatric Use
In controlled Phase 2/3 clinical studies (n=1975), Exubera was administered to 266 patients ≥65 years of age and 30 patients ≥75 years of age. The majority of these patients had type 2 diabetes. The change in HbA1C and rate of hypoglycemia did not differ by age.
Adverse Reactions
The safety of Exubera alone, or in combination with subcutaneous insulin or oral agents, has been evaluated in approximately 2500 adult patients with type 1 or type 2 diabetes who were exposed to Exubera. Approximately 2000 patients were exposed to Exubera for greater than 6 months and more than 800 patients were exposed for more than 2 years.
Non-Respiratory Adverse Events
Non-respiratory adverse events reported in ≥1% of 1977 Exubera-treated patients in controlled Phase 2/3 clinical studies, regardless of causality, include (but are not limited to) the following:
Metabolic and Nutritional: hypoglycemia (see WARNINGS and PRECAUTIONS)
Body as a whole: chest pain
Digestive: dry mouth
Special senses: otitis media (type 1 pediatric diabetics)
Hypoglycemia
The rates and incidence of hypoglycemia were comparable between Exubera and subcutaneous regular human insulin in patients with type 1 and type 2 diabetes. In type 2 patients who were not adequately controlled with single oral agent therapy, the addition of Exubera was associated with a higher rate of hypoglycemia than was the addition of a second oral agent.
Chest pain
A range of different chest symptoms were reported as adverse reactions and were grouped under the non-specific term chest pain. These events occurred in 4.7% of Exubera-treated patients and 3.2% of patients in comparator groups. The majority (>90%) of these events were reported as mild or moderate. Two patients in the Exubera and one in the comparator group discontinued treatment due to chest pain. The incidence of all-causality adverse events related to coronary artery disease, such as angina pectoris or myocardial infarction was comparable in the Exubera (0.7% angina pectoris; 0.7% myocardial infarction) and comparator (1.3% angina pectoris; 0.7% myocardial infarction) treatment groups.
Dry Mouth
Dry mouth was reported in 2.4% of Exubera-treated patients and 0.8% of patients in comparator groups. Nearly all (>98%) of dry mouth reported was mild or moderate. No patients discontinued treatment due to dry mouth.
Ear Events in Pediatric Diabetics
Pediatric type 1 diabetics in Exubera groups experienced adverse events related to the ear more frequently than did pediatric type 1 diabetics in treatment groups receiving only subcutaneous insulin. These events included otitis media (Exubera 6.5%; SC 3.4%), ear pain (Exubera 3.9%; SC 1.4%), and ear disorder (Exubera 1.3%; SC 0%).
Respiratory Adverse Events
Table 6 shows the incidence of respiratory adverse events for each treatment group that were reported in ≥1% of any treatment group in controlled Phase 2 and 3 clinical studies, regardless of causality.
Table 6: Respiratory Adverse Events Reported in ≥1% of Any Treatment Group in Controlled Phase 2 and 3 Clinical Studies, Regardless of Causality
| Percent of Patients Reporting Event | |||||
|---|---|---|---|---|---|
| Adverse Event | Type 1 Diabetes | Type 2 Diabetes | |||
| Exubera N = 698 | SC N = 705 | Exubera N = 1279 | SC N = 488 | OAs N = 644 | |
| SC = subcutaneous insulin comparator; OA = oral agent comparators | |||||
| Respiratory Tract Infection | 43.3 | 42.0 | 29.2 | 38.1 | 19.7 |
| Cough Increased | 29.5 | 8.8 | 21.9 | 10.2 | 3.7 |
| Pharyngitis | 18.2 | 16.6 | 9.5 | 9.6 | 5.9 |
| Rhinitis | 14.5 | 10.9 | 8.8 | 10.5 | 3.0 |
| Sinusitis | 10.3 | 7.4 | 5.4 | 10.0 | 2.3 |
| Respiratory Disorder | 7.4 | 4.1 | 6.1 | 10.2 | 1.7 |
| Dyspnea | 4.4 | 0.9 | 3.6 | 2.5 | 1.4 |
| Sputum Increased | 3.9 | 1.3 | 2.8 | 1.0 | 0.5 |
| Bronchitis | 3.2 | 4.1 | 5.4 | 3.9 | 4.0 |
| Asthma | 1.3 | 1.3 | 2.0 | 2.3 | 0.5 |
| Epistaxis | 1.3 | 0.4 | 1.2 | 0.4 | 0.8 |
| Laryngitis | 1.1 | 0.4 | 0.5 | 0.4 | 0.3 |
| Pneumonia | 0.9 | 1.1 | 0.9 | 1.6 | 0.6 |
| Voice Alteration | 0.1 | 0.1 | 1.3 | 0.0 | 0.3 |
Cough
In 3 clinical studies, patients who completed a cough questionnaire reported that the cough tended to occur within seconds to minutes after Exubera inhalation, was predominantly mild in severity and was rarely productive in nature. The incidence of this cough decreased with continued Exubera use. In controlled clinical studies, 1.2% of patients discontinued Exubera treatment due to cough.
Dyspnea
Nearly all (>97%) of dyspnea was reported as mild or moderate. A small number of Exubera-treated patients (0.4%) discontinued treatment due to dyspnea compared to 0.1% of comparator-treated patients.
Other Respiratory Adverse Events - Pharyngitis, Sputum Increased and Epistaxis
The majority of these events were reported as mild or moderate. A small number of Exubera-treated patients discontinued treatment due to pharyngitis (0.2%) and sputum increased (0.1%); no patients discontinued treatment due to epistaxis.
Pulmonary Function
The effect of Exubera on the respiratory system has been evaluated in over 3800 patients in controlled phase 2 and 3 clinical studies (in which 1977 patients were treated with Exubera). In randomized, open-label clinical trials up to two years duration, patients treated with Exubera demonstrated a greater decline in pulmonary function, specifically the forced expiratory volume in one second (FEV1) and the carbon monoxide diffusing capacity (DLCO), than comparator treated patients. The mean treatment group differences in FEV1 and DLCO, were noted within the first several weeks of treatment with Exubera, and did not progress over the two year treatment period. In one completed controlled clinical trial in patients with type 2 diabetes following two years of treatment with Exubera, patients showed resolution of the treatment group difference in FEV1 six weeks after discontinuation of therapy. Resolution of the effect of Exubera on pulmonary function in patients with type 1 diabetes has not been studied after long-term treatment.
Figures 3 through 6 display the mean FEV1 and DLCO change from baseline versus time from two ongoing randomized, open-label, two year studies in 580 patients with type 1 and 620 patients with type 2 diabetes.
Figure 3: Change from Baseline FEV1 (L) in Patients with Type 1 Diabetes (Mean +/-Standard Deviation)
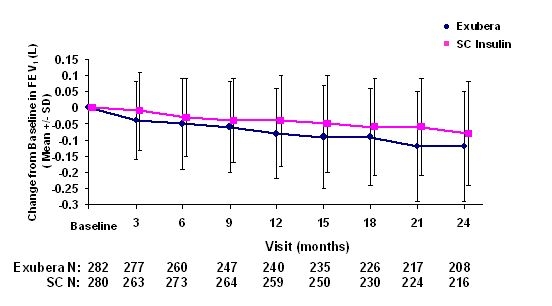
Figure 4: Change from Baseline FEV1 (L) in Patients with Type 2 Diabetes (Mean +/- Standard Deviation)
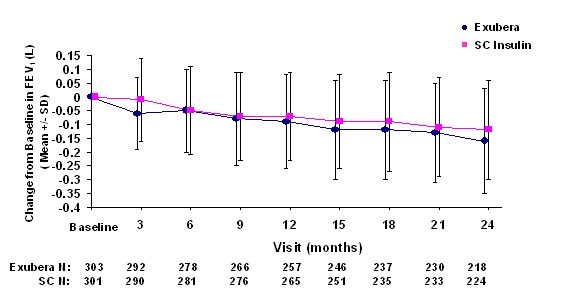
Following 2 years of Exubera treatment in patients with type 1 and type 2 diabetes, the difference between treatment groups for the mean change from baseline FEV1 was approximately 40 mL, favoring the comparator.
Figure 5: Change from Baseline DLco (mL/min/mmHg) in Patients with Type 1 Diabetes (Mean +/- Standard Deviation)
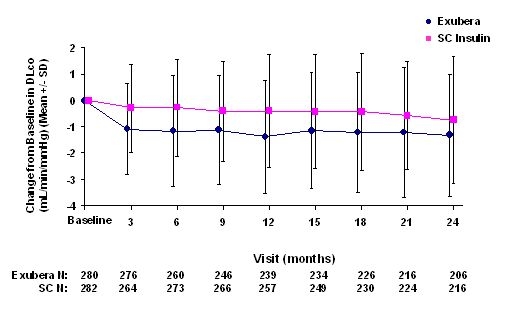
Figure 6: Change from Baseline DLco (mL/min/mmHg) in Patients with Type 2 Diabetes (Mean +/- Standard Deviation)
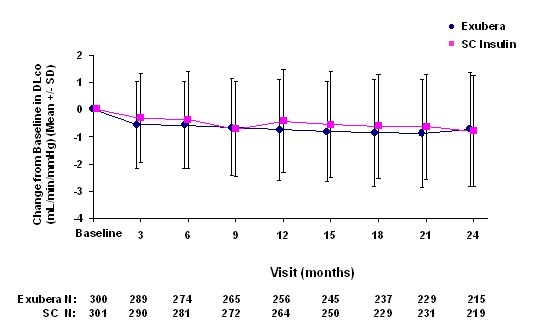
Following 2 years of Exubera treatment, the difference between treatment groups for the mean change from baseline DLCO was approximately 0.5mL/min/mmHg (type 1 diabetes), favoring the comparator, and approximately 0.1mL/min/mmHg (type 2 diabetes), favoring Exubera.
During the two-year clinical trials, individual patients experienced notable declines in pulmonary function in both treatment groups. A decline from baseline FEV1 of ≥ 20% at last observation occurred in 1.5% of Exubera-treated and 1.3% of comparator-treated patients. A decline from baseline DLCO of ≥ 20% at last observation occurred in 5.1% of Exubera-treated and 3.6% of comparator treated patients.
Overdosage
Hypoglycemia may occur as a result of an excess of insulin relative to food intake, energy expenditure, or both.
Mild to moderate episodes of hypoglycemia usually can be treated with oral glucose. Adjustments in drug dosage, meal patterns, or exercise, may be needed.
Severe episodes of hypoglycemia with coma, seizure, or neurologic impairment may be treated with intramuscular/subcutaneous glucagon or concentrated intravenous glucose. Sustained carbohydrate intake and observation may be necessary because hypoglycemia may recur after apparent clinical recovery.
Dosage and Administration
Exubera, like rapid-acting insulin analogs, has a more rapid onset of glucose-lowering activity compared to subcutaneously injected regular human insulin. Exubera has a duration of glucose-lowering activity comparable to subcutaneously injected regular human insulin and longer than rapid-acting insulin. Exubera doses should be administered immediately prior to meals (no more than 10 minutes prior to each meal).
In patients with type 1 diabetes, Exubera should be used in regimens that include a longer-acting insulin. For patients with type 2 diabetes, Exubera may be used as monotherapy or in combination with oral agents or longer-acting insulin.
Because of the effect of Exubera on pulmonary function, all patients should have pulmonary function assessed prior to initiating therapy with Exubera. Periodic monitoring of pulmonary function is recommended for patients being treated with Exubera (see PRECAUTIONS, Pulmonary Function).
Exubera is intended for administration by inhalation and must only be administered using the Exubera® Inhaler. Refer to the Exubera Medication Guide for a description of the Exubera® Inhaler and for instructions on how to use the inhaler.
Calculation of Initial Pre-Meal Exubera Dose
The initial dosage of Exubera should be individualized and determined based on the physician's advice in accordance with the needs of the patient. Recommended initial pre-meal doses are based on clinical trials in which patients were requested to eat three meals per day. Initial pre-meal doses may be calculated using the following formula: [Body weight (kg) X 0.05 mg/kg = pre-meal dose (mg)] rounded down to the nearest whole milligram number (e.g., 3.7 mg rounded down to 3 mg).
Approximate guidelines for initial, pre-meal Exubera doses, based on patient body weight, are indicated in Table 7:
Table 7: Approximate Guidelines for Initial, Pre-Meal Exubera Dose (based on patient body weight)
| Patient Weight (in kg) | Patient Weight (in lb) | Initial Dose per Meal | Number of 1 mg Blisters per Dose | Number of 3 mg Blisters per Dose |
|---|---|---|---|---|
| 30 to 39.9 kg | 66 - 87 lb | 1 mg per meal | 1 | - |
| 40 to 59.9 kg | 88 - 132 lb | 2 mg per meal | 2 | - |
| 60 to 79.9 kg | 133 - 176 lb | 3 mg per meal | - | 1 |
| 80 to 99.9 kg | 177 - 220 lb | 4 mg per meal | 1 | 1 |
| 100 to 119.9 kg | 221- 264 lb | 5 mg per meal | 2 | 1 |
| 120 to 139.9 kg | 265 - 308 lb | 6 mg per meal | - | 2 |
A 1 mg blister of Exubera inhaled insulin is approximately equivalent to 3 IU of subcutaneously injected regular human insulin. A 3 mg blister of Exubera inhaled insulin is approximately equivalent to 8 IU of subcutaneously injected regular human insulin. Table 8 provides the approximate IU dose of regular subcutaneous human insulin for Exubera inhaled insulin doses from 1 mg to 6 mg.
Table 8: Approximate Equivalent IU Dose of Regular Human Subcutaneous Insulin for Exubera Inhaled Insulin Doses Ranging from 1 mg to 6 mg
| Dose (mg) | Approximate Regular Insulin SC Dose in IU | Number of 1 mg Exubera Blisters per Dose | Number of 3 mg Exubera Blisters per Dose |
|---|---|---|---|
| 1 mg | 3 | 1 | - |
| 2 mg | 6 | 2 | - |
| 3 mg | 8 | - | 1 |
| 4 mg | 11 | 1 | 1 |
| 5 mg | 14 | 2 | 1 |
| 6 mg | 16 | - | 2 |
Patients should combine 1 mg and 3 mg blisters so that the least number of blisters per dose are taken (e.g., a 4 mg dose should be administered as one 1 mg blister and one 3 mg blister). Consecutive inhalation of three 1 mg unit dose blisters results in significantly greater insulin exposure than inhalation of one 3 mg unit dose blister. Therefore, three 1 mg doses should not be substituted for one 3 mg dose (see CLINICAL PHARMACOLOGY, Pharmacokinetics). When a patient is stabilized on a dosing regimen that includes 3 mg blisters, and the 3 mg blisters become temporarily unavailable, the patient can temporarily substitute two 1 mg blisters for one 3 mg blister. Blood glucose should be monitored closely.
As with all insulins, additional factors that should be taken into consideration when determining the Exubera starting dose include, but are not limited to, patient's current glycemic control, previous response to insulin, duration of diabetes, and dietary and exercise habits.
Considerations for Dose Titration
After initiating Exubera therapy, as with other glucose-lowering agents, dose adjustment may be required based on the patient's need (e.g., blood glucose concentrations, meal size and nutrient composition, time of day and recent or anticipated exercise). Each patient should be titrated to their optimal dosage based on blood glucose monitoring results.
As for all insulins, the time course of Exubera action may vary in different individuals or at different times in the same individual.
Exubera may be used during intercurrent respiratory illness (e.g., bronchitis, upper respiratory tract infection, rhinitis). Close monitoring of blood glucose concentrations and dose adjustment may be required on an individual basis. Inhaled medicinal products (e.g. bronchodilators) should be administered prior to administration of Exubera.
How Supplied
Exubera (insulin human [rDNA origin]) Inhalation Powder is available in 1 mg and 3 mg unit dose blisters. The blisters are dispensed on perforated cards of six unit dose blisters (PVC/Aluminum). The two strengths are differentiated by color print and tactile marks that can be differentiated by touch. The 1 mg blisters and respective perforated cards are printed with green ink and the cards are marked with one raised bar. The 3 mg blisters and respective perforated cards are printed with blue ink and the cards are marked with three raised bars.
Five blister cards are packaged in a clear plastic (PET) thermoformed tray. Each PET tray also contains a desiccant and is covered with a clear plastic (PET) lid. The tray of five blister cards (30 unit dose blisters) is sealed in a foil laminate pouch with a desiccant.
Exubera (insulin human [rDNA origin]) Inhalation Powder blisters, an Exubera® Inhaler, and replacement Exubera® Release Units are required to initiate therapy with Exubera and are provided in the Exubera Kit. A fully assembled Exubera® Inhaler consists of the inhaler base, a chamber, and an Exubera® Release Unit. A fully assembled Inhaler is packaged with a replacement Chamber and is available in the Exubera Kit and as a separate unit. The Chamber is also available as an individual component.
Exubera® Release Units are individually packaged in a sealed thermoformed tray. One Exubera® Release Unit is included in each fully assembled Inhaler. Two additional Release Units are provided in the Exubera Kit and in each Combination Pack. Exubera Release Units are also available individually.
See Tables 9 and 10 for a description of these configurations.
Table 9
| Exubera (insulin human [rDNA origin]) Inhalation Powder is available as follows: | ||
|---|---|---|
| Description | Contents | NDC |
| Exubera KIT | 1 Exubera Inhaler 1 Replacement Chamber 1 mg × 180 blisters 3 mg × 90 blisters 2 Exubera® Release Units | 0069-0050-85 |
| Exubera Combination Pack 12 | 1 mg × 90 blisters 3 mg × 90 blisters 2 Exubera® Release Units | 0069-0050-19 |
| Exubera Combination Pack 15 | 1 mg × 180 blisters 3 mg × 90 blisters 2 Exubera® Release Units | 0069-0050-53 |
| Exubera 1 mg Patient Pack | 90 × 1 mg 2 Exubera® Release Units | 0069-0707-37 |
| Exubera 3 mg Patient Pack | 90 × 3 mg 2 Exubera® Release Units | 0069-0724-37 |
Table 10
| Exubera® Inhaler and Components are available as follows: | ||
|---|---|---|
| Description | Contents | NDC |
| Exubera® Inhaler & Chamber | 1 Exubera® Inhaler 1 Replacement Chamber | 0069-0054-19 |
| Exubera® Release Units | 2 Exubera® Release Units | 0069-0097-41 |
| Exubera® Chamber | 1 Replacement Chamber | 0069-0061-19 |
Blister Storage
Not in-use (Unopened): Store at controlled room temperature, 25°C (77°F); excursions permitted to 15-30°C (59-86°F) [see USP Controlled Room Temperature]. Do not freeze. Do not refrigerate.
In-use: Once the foil overwrap is opened, unit dose blisters should be protected from moisture, stored at 25°C (77°F); excursions permitted to 15-30°C (59-86°F) [see USP Controlled Room Temperature]. Do not freeze. Do not refrigerate. Unit dose blisters should be used within 3 months after opening the foil overwrap. Return the blisters to the overwrap to protect from moisture. Additional care should be taken to avoid humid environments, e.g. steamy bathroom following a shower.
Discard blister if frozen.
Inhaler Storage
Store at controlled room temperature, 25°C (77°F); excursions permitted to 15-30°C (59-86°F) [see USP Controlled Room Temperature]. Do not freeze. Do not refrigerate.
The Exubera® Inhaler can be used for up to 1 year from the date of first use.
Replacing The Exubera® Release Unit
The Exubera® Release Unit in the Exubera® Inhaler should be changed every 2 weeks.
Keep out of reach of children
Rx only

LAB-0331-12.0
last revision 04/2008
Exubera, insulin human [rDNA origin] Patient information (in plain English)
Detailed Info on Signs, Symptoms, Causes, Treatments of Diabetes
The information in this monograph is not intended to cover all possible uses, directions, precautions, drug interactions or adverse effects. This information is generalized and is not intended as specific medical advice. If you have questions about the medicines you are taking or would like more information, check with your doctor, pharmacist, or nurse.
back to: Browse all Medications for Diabetes
APA Reference
Staff, H.
(2008, April 1). Exubera for Treatment of Diabetes - Exubera Full Prescribing Information, HealthyPlace. Retrieved
on 2026, March 4 from https://www.healthyplace.com/diabetes/medications/exubera-inhaler-prescribing-information